Textbook Questions and Answers
1. In the paragraph below write ‘solid’, ‘liquid’ or ‘gas’ in each of the blank (brackets) depending on the substance referred to just before.
Question a.
On a bright sunny day, Riya and Gargi are playing with a ball (…..) in the park. Gargi feels thirsty. So, Riya brings tender coconut water (…..) for her. At the same time, a strong breeze (…..) starts blowing and it also begins to rain (…..). They run back into the house (…..), change their clothes (…..) and then their mother gives them a cup (…..) of hot milk (…..) to drink.
Answer:
solid, liquid, gas, liquid, solid, solid, solid, liquid.
2. Discuss.
Question a.
Riya pours some water from her bottle into another bottle. Does it change the shape of the water?
Answer:
Yes, the shape of water changes as water is in liquid state. Liquids do not have a shape of its own. They take the shape of the container.
Question b.
Halima picks up a small stone from the ground and puts it in the water in a dish. Does the shape of the stone change?
Answer:
No, the shape of the stone does not change. Stone is a solid, hence retains its shape.
3. Write the properties of these substances.
(water, glass, chalk, iron ball, sugar, salt, flour, coal, soil, pen, ink, soap)
Question a.
Write the properties of these substances.
(water, glass, chalk, iron ball, sugar, salt, flour, coal, soil, pen, ink, soap)
Answer:
Properties of substances:
| Substance | State | Properties |
| 1. Water | Liquid | Fluidity, density, solubility, transparency, thermal conductivity. |
| 2. Glass | Solid | Brittleness, hardness, density, transparency. |
| 3. Chalk | Solid | Brittleness, density. |
| 4. Iron ball | Solid | Hardness, density, malleability, ductility, electrical ductility, conductivity, thermal conductivity, luster, sonority. |
| 5. Sugar | Solid | Brittleness, density, solubility. |
| 6. Salt | Solid | Brittleness, density, solubility. |
| 7. Flour | Solid | Density, solubility. |
| 8. Coal | Solid | Brittleness, density, thermal conductivity. |
| 9. Soil | Solid | Brittleness, density. |
| 10. Pen | Solid | Hardness, density. |
| 11. Ink | Liquid | Fluidity, density, solubility. |
| 12. Soap | Solid | Brittleness, hardness, density, solubility. |
4. What is sublimation? Write the names of everyday substances that sublimate.
Question a.
What is sublimation? Write the names of everyday substances that sublimate.
Answer:
- The change of a solid substance directly into a gas or vapour without first changing into liquid is called sublimation.
- Substances that sublimate: Camphor, napthalene balls, ammonium chloride, iodine.
5. What is made from? Why?
a. A sickle to cut sugarcane.
b. The sheets used for roofing.
c. A screwdriver
d. A pair of tongs.
e. Electric cables.
f. Ornaments.
g. Pots and pans.
Question a.
A sickle to cut sugarcane.
Answer:
A sickle is made of iron. An iron sickle is hard and malleable. When sharpened it will be able to cut the hard sugarcane.
Question b.
The sheets used for roofing:
Answer:
- The sheets used for roofing are made of plastic, aluminium.
- Plastic is hard, hence, protects against weather conditions.
- Plastic is transparent, hence, sunlight can pass through it.
- Aluminium is hard, light weight and durable, hence, protects against all weather conditions.
- Malleable hence formed into thin sheets.
Question c.
A screwdriver:
Answer:
- A screwdriver is made up of iron, steel, aluminium.
- A screwdriver possesses property of hardness hence, it easily pierces a screw in piece of wood, wall, metals etc.
Question d.
A pair of tongs:
Answer:
- A pair of tongs are made up of iron, steel aluminium etc. Tongs are used to lift hot, boiling utensils or vessels.
- Tongs are hard, ductile and malleable.
- Hence, have strong grip to hold utensils.
- Rubbers fitted on the ends will protect from thermal conduction, from bums.
Question e.
Electric cables:
Answer:
- Electric cables are metal wires (thin) wound in plastic.
- Metal wires possess the property of hardness, ductility, electrical conductivity.
- Plastic /rubber covering possesses the property of hardness, elasticity and are bad conductors of heat and electricity.
Question f.
Ornaments:
Answer:
- They are made up of metals like gold and silver.
- They possess the property of hardness, ductility, malleability, lustre.
Question g.
Pots and pans: Answer:
- They are used to cook food, hence metals like aluminium, steel are used.
- They possess the property of hardness, ductility, malleability, thermal conductivity, electrical conductivity, (microwave ovens)
6. What will happen if ….? And why?
Question a.
Nails are made of plastic
Answer:
If nails are made of plastic, they will not be able to pierce through other substances on being pushed or forced by a hammer. Plastic lacks the property of hardness.
Question b.
A bell is made of wood.
Answer:
- If a bell is made of wood it will never make a ringing sound. A wooden bell does not have the property of being sonorous.
- Sonority is the property of metals to produce a ringing sound.
Question c.
Rubber is not fitted on a pair of tongs.
Answer:
- Rubber is a bad conductor of heat and electricity. It will not allow heat to pass to the hands/handle of the tongs, thus protecting us.
- Pair of tongs are made up of metals which conduct heat and electricity. They have file property of thermal conduction and electrical conduction.
- If rubber is not fitted on a pair of tongs, we will not be able to lift hot objects with it.
Question d.
A knife is made of wood.
Answer:
Wood does not have the property of malleability. Therefore, the edge of wooden knife will be blunt. Hence, we will not be able to cut anything with it.
Question e.
An axe is made of rubber.
Answer:
- If an axe is made of rubber, it will not be used to cut wood or tree.
- Rubber does not have the property of hardness that is required to push through to cut it.
7. Who am I?
Question a.
I’m found in a thermometer, I measure your temperature.
Answer:
Mercury
Question b.
I make things hot or cold.
Answer:
Heat
Question c.
I have no shape whatsoever!
Answer:
Liquid, gases
Question d.
I dissolve in water, but not in kerosene.
Answer:
Salt
8. Why does this happen?
Question a.
Coconut oil thickens in winter.
Answer:
Coconut oil is in liquid state. In winter the surrounding temperature / atmospheric temperature starts decreasing. Coconut oil starts cooling or losing heat, it changes to solid state.
Thus coconut oil thickens in winter.
Question b.
Kerosene left open in a dish disappears.
Answer:
When kerosene is left open in a dish, it is exposed to surrounding temperature. As the temperature is more, kerosene starts continuously evaporating and finally disappears.
Question c.
The fragrance of incense sticks lighted in one corner of a room spreads to the other corner.
Answer:
The fragrance of incense sticks is given out in the form of scented vapours. As vapours are in gaseous state, the gas molecules spread out in the room. The molecules of gas move very fast and there are no forces to stop them from going apart. Therefore the fragrance of incense sticks lighted in one corner of room spreads to the other corner.
Question d.
What you see in the picture.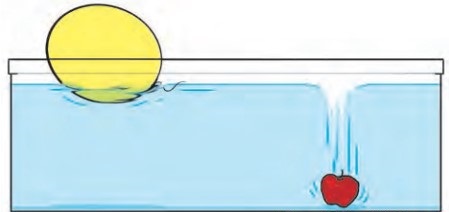
Answer:
The mass of plastic ball is less than an apple. This difference is because of their densities. Since an apple has greater density, it will sink to the bottom on other hand the plastic ball has lesser density, it will float over water surface.
Activity:
Question 1.
Find out how the big statues of wax are made.
Question 2.
Visit a jeweller’s shop and find out how ornaments are made.
Important Questions and Answers
Fill in the blanks:
Question 1.
The state of a substance changes if it is …………… or …………… .
Answer:
heated, cooled
Question 2.
Every substance in our surroundings is found in either the ……………, …………… or gaseous state.
Answer:
solid, liquid
Question 3.
On …………… heat, the substance changes from solid to liquid and liquid to gas.
Answer:
gaining
Question 4.
When the substance cools, or …………… heat, it changes from gaseous to liquid and liquid to solid state.
Answer:
loses
Question 5.
A …………… amount of heat must be gained or lost before the state of a substance can change.
Answer:
specific
Question 6.
When a substance gets heat, it becomes …………… and then …………… .
Answer:
warm, hot
Question 7.
If the substance is very hot, we could get …………… .
Answer:
scalded
Question 8.
A thermometer is used to measure …………… .
Answer:
temperature
Question 9.
…………… is the unit of measuring temperature.
Answer:
Degree Celsius (°C)
Question 10.
Nowadays, …………… thermometers are frequently used.
Answer:
digital
Question 11.
Solids have a shape of its …………… .
Answer:
own
Question 12.
Solids have a …………… volume.
Answer:
definite
Question 13.
Liquids take the shape of the …………… .
Answer:
container
Question 14.
Liquids have a …………… volume.
Answer:
specific
Question 15.
Air occupies all the available …………… .
Answer:
space
Question 16.
Evaporation occurs from the …………… of the water.
Answer:
surface
Question 17.
At sea-level, pure water boils at …………… .
Answer:
100°C
Question 18.
Condensation of steam takes place at …………… .
Answer:
100°C
Question 19.
The temperature of a substance can fall below …………… .
Answer:
0°C
Question 20.
Ice melts at …………… .
Answer:
0°C
Question 21.
Each substance has a specific boiling point which is also its …………… point.
Answer:
condensation
Question 22.
Each substance has a specific melting point which is the same as its …………… point.
Answer:
freezing
Question 23.
Candles are made by melting …………… wax.
Answer:
paraffin
Question 24.
Solid carbon-dioxide is …………… .
Answer:
dry ice
Question 25.
Liquid …………… is used in animal husbandry.
Answer:
nitrogen
Question 26.
Sand is melted to make …………… .
Answer:
glass
Question 27.
Iron is melted to make …………… .
Answer:
tools
Question 28.
Substances can be identified by studying their …………… .
Answer:
properties
Question 29.
Substances that break into small particles are said to be …………… .
Answer:
brittle
Question 30.
The …………… of any liquid is determined by how easily it flows.
Answer:
fluidity
Question 31.
Between substances of the same volume, the ones with greater density are …………… than those of lesser density.
Answer:
heavier
Question 32.
The property of a substance of getting …………… is called its solubility.
Answer:
dissolved
Question 33.
Minerals from the earth’s crust are …………… to obtain metals.
Answer:
processed
Question 34.
Metals can be converted into …………… by hammering.
Answer:
sheets
Question 35.
Metals can be stretched and drawn into …………… .
Answer:
wires
Question 36.
All metals are …………… of electricity to a greater or lesser extent.
Answer:
conductors
Question 37.
Every metal has a …………… colour by which it can be identified.
Answer:
specific
Question 38.
Metals produce …………… sound.
Answer:
ringing
Question 39.
Metals form a …………… group of substances.
Answer:
separate
Question 40.
Heat is the cause of the change of the state of …………… .
Answer:
substances
Match the columns:
Question 1.
| Column ‘A’ | Column ‘B’ |
| 1. Boiling water | a. > 35° C |
| 2. Body temperature | b. 0° C |
| 3. Freezing water | c. < 5° C |
| 4. Air (summer afternoon) | d. < 15° C |
| 5. Inside a fridge | e. < -18° C |
| 6. Air (winter night) | f. 100° C |
| 7. Inside the freezer | g. 37°C |
Answer:
| Column ‘A’ | Column ‘B’ |
| 1. Boiling water | f. 100° C |
| 2. Body temperature | g. 37°C |
| 3. Freezing water | b. 0° C |
| 4. Air (summer afternoon) | a. > 35° C |
| 5. Inside a fridge | c. < 5° C |
| 6. Air (winter night) | d. < 15° C |
| 7. Inside the freezer | e. < -18° C |
Answer in one sentence:
Question 1.
What is change of state of substances?
Answer:
When a substance changes from one state to another, the process is called change of state of the substance.
Question 2.
When does state of substance change?
Answer:
State of substance changes when it is heated or cooled.
Question 3.
In which state do substances exist in our surroundings?
Answer:
The substances exist in solid, liquid and gaseous form in our surroundings.
Question 4.
What happens when a substance gains heat?
Answer:
When a substance gains heat, it changes its state i.e. from solid to liquid and liquid to gas.
Question 5.
What happens when a substance loses heat?
Answer:
When a substance loses heat, it changes its state from gaseous to liquid and liquid to solid state.
Question 6.
How do we tell how hot or cold a substance is?
Answer:
The temperature on the thermometer will tell us how hot or cold a substance is.
Question 7.
What is the unit of measuring temperature.
Answer:
Degrees Celsius (°C) is the unit of measuring temperature.
Question 8.
What is the boiling point of water?
Answer:
The boiling point of water is 100° C.
Question 9.
What is condensation?
Answer:
When vapour cools, it is converted into liquid again. This process is condensation.
Question 10.
At what temperature condensation of steam takes place?
Answer:
Condensation of steam takes place at 100° C.
Question 11.
What is the freezing point of water?
Answer:
0° C is the freezing point of water.
Question 12.
What is the temperature of air in the freezer of a refrigerator?
Answer:
-18° C is the temperature of air in the freezer of a refrigerator.
Question 13.
At what temperature ice melts?
Answer:
Ice melts at 0° C.
Question 14.
How are candles made?
Answer:
Candles are made by melting paraffin wax.
Question 15.
What is the use of solid carbon-dioxide?
Answer:
Solid carbon-dioxide (dry ice) is used to make ice cream and to keep it frozen.
Question 16.
What is the use of liquid nitrogen?
Answer:
Liquid nitrogen is used in animal husbandry.
Question 17.
What is sublimation?
Answer:
The change of a solid substance directly into gas or vapour without changing into a liquid is called sublimation.
Question 18.
Define brittleness / What is brittleness?
Answer:
Some substances break into small pieces or particles. Such substances are said to be brittle. This property of substances is called brittleness.
Question 19.
Define hardness / What is hardness?
Answer:
The hardness of a substance is determined by how much resistance it offers to the substances being pushed through it.
Question 20.
Define elasticity / What is elasticity?
Answer:
Some substances change their shape when a force is applied on them but return to their original shape and size when the force is removed. This property is called elasticity.
Question 21.
Define fluidity / What is fluidity?
Answer:
Liquids flow downward on a sloping surface. This property is called fluidity.
Question 22.
How is fluidity of any liquid determined?
Answer:
Fluidity of any liquid is determined by how easily it flows.
Question 23.
Define density / What is density?
Answer:
The mass of different substances having the same volume can be different. This difference is because of the difference in their densities. Between substances of the same volume, the ones with greater density are heavier than those of lesser density.
Question 24.
Define solubility / What is solubility?
Answer:
The property of a substance of getting dissolved is called its solubility.
Question 25.
Define transparency / What is transparency?
Answer:
When we can look through a substance and see things on the other side, then that substance is said to be transparent. This property of the substances is called transparency.
Question 26.
List some transparent substances.
Answer:
Glass, air, clean water and some types of plastic are transparent substances.
Question 27.
What are metals?
Answer:
Metals are substances like copper, gold, iron, aluminium. They are found in the form of minerals deep inside the earth. Minerals from the earth’s crust are processed to obtain metals.
Question 28.
Define malleability. / What is malleability?
Answer:
Metals can be converted into sheets by hammering. This property of metals is called malleability.
Question 29.
Define ductility. / What is ductility?
Answer:
Metals can be stretched and drawn into thin wires. This property of metals is called ductility
Question 30.
Name some ductile metals.
Answer:
Metals like silver, gold, platinum can be drawn into fine wires.
Question 31.
Define electrical conductivity. / What is electrical conduction?
Answer:
Electricity flows through metals. All metals are conductors of electricity to a greater or lesser extent.
Question 32.
Define thermal conductivity. / What is thermal conductivity?
Answer:
Metals allow heat to flow through them. This property is called thermal conductivity
Question 33.
What is lustre?
Answer:
The typical shine or characteristic colour by which metal can be identified is called lustre.
Question 34.
What is sonority of metals? / Define sonority.
Answer;
Metals produce a ringing sound. This property is called the sonority of metals.
Give scientific reasons for following:
Question 1.
Metals are used to make musical instruments.
Answer:
Metals possess the property of being sonorous, i.e. produce a ringing sound. Hence, they are used to make musical instruments.
Question 2.
Ornaments are made up of metals.
Answer:
Metals have the property of being malleable, ductile, lustrous. Hence, ornaments are made up of metals.
Question 3.
Why should we not put our hand or finger in the water to judge the hotness of water?
Answer:
We should never put our hand or finger in the water to judge how hot it is because that is not an accurate measure. Besides if the substance is very hot, we could get scalded.
Can you tell?
Question 1.
Why are electric boards fitted on the wall made of plastic or wood?
Answer:
Plastic or wood are bad conductors of heat and electricity. Electric boards are made up of plastic or wood. So that while touching we will not get electric shock.
Question 2.
The handle of cooker is made of plastic. Why?
Answer:
Cooker is made of metal. When food is cooked in it, it gets heated and the whole of its body becomes hot due to thermal conductivity. Hence with the plastic handle we can easily lift the hot cooker as plastic is a bad conductor of heat.
Question 3.
Use your brain power!
On opening a box of camphor, its smell spreads all around. Why does this happen?
Answer:
- Camphor is a sublimate substance.
- When a box of camphor is opened it changes its state from solid to gas or vapour state.
- This change takes due to the process of sublimation where camphor absorbs heat from surrounding to change from solid to gaseous state.
- Camphor particles in gaseous state start spreading all around.
- Hence on opening a box of camphor its smell spreads all around.
Question 4.
Identify the objects shown in fig. 5.14. From which substances are they made? What are these substances called as a group?
Answer:
In fig 5.14, the metallic bars are shown, these bars are made up of shiny solid. These substances are called metals.
Question 5.
Name the solid, liquid and gaseous states of water.
Answer:
- Solid- Ice
- Liquid – water
- Gas – water vapour.
Question 6.
Read this list of substances:
spirit, camphor, petrol, ghee, coconut oil, naphthalene balls, ammonium chloride (navsagar).
Question a.
Which one freezes in winter?
Answer:
Coconut oil, ghee.
Question b.
Which liquids have you seen change into a vapour?
Answer:
Spirit, petrol.
Question c.
Which solid directly changes into gaseous state?
Answer:
Camphor, naphthalene balls, ammonium chloride.
Question 7.
The chart given below shows the boiling point and freezing point of some substances. State whether these substances are solid, liquid or gaseous at room temperature.
| Substance | Freezing Point | Boiling Point |
| Candle | 60 °C | 350 °C |
| Plastic | > 250 °C | 954 °C |
| Iron | 1535 °C | 2862 °C |
Answer:
Candle, plastic and iron are in solid state at room temperature.
Distinguish between solids, liquids and gases.
Answer:
| Solids | Liquid Gases | |
| e.g.: A piece of iron | e.g.: Water, spirit, oil | e.g.: Air |
| Has a shape of its own, Retains shape, no matter how it is kept. | Does not have a shape of its own. Takes the shape of the container. | Does not have a shape of its own. Occupies all the available space. |
| Has a definite volume. Solids like sugar, sand when poured on a flat surface, form a heap. | It has a specific volume. Occupies definite portion of a container. Spreads on a flat surface on pouring. Flows downwards along a slope. Takes the shape of the surface. | Does not have a definite volume. On changing the pressure on a gas in a closed container, its volume also changes. |
Distinguish between Boiling and Melting.
Answer:
| Boiling | Melting |
| 1. When heat is supplied to liquids, they boil. | 1. When heat is supplied to solids, they melt. |
| 2. Boiling leads liquids to vapour/gaseous state. | 2. Melting leads solids to liquid state. |
| 3. The temparature at which liquid starts boiling continuously is called boiling point. | 3. The temperature at which solid turns to liquid completely is called melting point. |
Answer the following briefly:
Question 1.
List properties of solids
Answer:
- Solids have its own shape i.e. Retains shape, no matter how it is kept.
- Solids have definite volume.
- e.g. Sand when poured on a flat surface form a heap.
Question 2.
List properties of liquids.
Answer:
- Liquid does not have a shape of its own. Takes the shape of the container.
- A liquid has a specific volume i.e. occupies definite portion of a container.
- Liquids: e.g. water, milk, kerosene.
Question 3.
List properties of gases.
Answer:
- Gases does not have a shape of its own.
- Occupies all the available space.
- Does not have a definite volume.
- e.g. Air.
Question 4.
Explain with example how liquids take the shape of the surface.
Answer:
Liquids have a specific volume. They occupy definite portion of a container. Liquids spreads on a flat surface on pouring. Liquids flow downwards along a slope.
Question 5.
Explain Ebullition.
Answer:
- As the water gets heated, its temperature increases and it evaporates at a faster and faster rate.
- When water kept on a stove attains a particular temperature or level of heat, then evaporation takes place in all parts of the body of water.
- Then we see water bubbles rising at a faster and faster rate to the surface and steam mixing in the air.
- This is called boiling of water or Ebullition.
Question 6.
Explain: Boiling point and condensation point of water are one and the same.
Answer:
Water boils at 100°C. i.e. boiling point of water is 100°C. Condensation of steam also takes place at 100° C. Thus boiling point and condensation point of water are one and the same.
Question 7.
Explain freezing point of water.
Answer:
- Water kept in a fridge or on ice becomes cooler and cooler i.e. its temperature falls.
- At a certain temperature water does not cool further but starts freezing and forms ice.
- The temperature at which this happens is called the freezing point of water. (0° C)
Question 8.
Explain: Freezing point and melting point of water are same.
Answer:
- The temperature at which water does not get any cooler but starts freezing and forming ice is 0° C.
- When ice gets heat, it starts melting or changes into liquid state at 0° C.
- Thus, freezing point and melting point of water are one and the same.
Question 9.
List various uses of changes in physical state.
Answer:
- Candles are made by melting paraffin wax.
- Solid carbon-dioxide (dry ice) is used to make ice-cream and to keep it frozen.
- Liquid nitrogen is used in animal husbandry.
- Sand (silica) is melted to make glass.
- Metals like gold and silver are melted to make ornaments.
- Iron is melted to make tools.
Question 10.
List the properties of substances:
Answer:
The properties of substances are
- Brittleness
- Hardness
- Elasticity
- Fluidity
- Density
- Solubility
- Transparency
Question 11.
List properties of metals:
Answer:
The properties of metals are
- Malleability
- Ductility
- Electrical conductivity
- Thermal conductivity
- Lustre
- Sonority
Question 12.
How can we change the volume of a gas?
Answer:
On changing the pressure on a gas in a closed container we can change its volume.
Can you tell?
Question 1.
Does water change into vapour the moment we place the vessel on a stove? Does water kept in fridge change at once into ice?
Answer:
No, it doesn’t. Water slowly changes from one state to another.
Question 2.
How do we tell how hot or cold a substance is?
Answer:
A thermometer is used to measure the temperature of the subatance which tell us hot or cold it is.
Question 3.
How will you identify the following
Question i.
A glass: Is it made of plastic, steel or glass?
Answer:
Glass is made of glass as it is transparent.
Question ii.
A rod: Iron or aluminium.
Answer:
A rod is made of iron as it is heavy.
Question iii.
A door: Wooden or glass?
Answer:
A door is wooden as it is opaque.
Question iv.
A white powder: Salt or chalk powder?
Answer:
If powder dissloves in water it is salt and if it does not dissolves in water it is chalk.
